

Sugar alcohols do not contribute to tooth decay in fact, xylitol deters tooth decay. Xylitol and lactitol are obtained similarly.Įrythritol is obtained by the fermentation of glucose and sucrose. More than a million tons of sorbitol are produced in this way every year. HOCH 2CH(OH)CH(OH)CH(OH)CH(OH)CHO + H 2 → HOCH 2CH(OH)CH(OH)CH(OH)CH(OH)CH HO H The conversion of glucose and mannose to sorbitol and mannitol is given as: Mannitol is no longer obtained from natural sources currently, sorbitol and mannitol are obtained by hydrogenation of sugars, using Raney nickel catalysts. sorbitol can be dehydrated to isosorbide). Unlike sugars, which tend to exist as rings, sugar alcohols do not-although they can be dehydrated to give cyclic ethers (e.g. They are further differentiated by the relative orientation ( stereochemistry) of these –OH groups. They have one –OH group attached to each carbon. Most have five- or six-carbon chains, because they are derived from pentoses (five-carbon sugars) and hexoses (six-carbon sugars), respectively. The sugar alcohols differ in chain length. In contrast, sugars have two fewer hydrogen atoms, for example HOCH 2(CHOH) nCHO or HOCH 2(CHOH) n−1C(O)CH 2OH. Sugar alcohols have the general formula HOCH 2(CHOH) nCH 2OH. Xylitol and sorbitol are popular sugar alcohols in commercial foods. In commercial foodstuffs, sugar alcohols are commonly used in place of table sugar ( sucrose), often in combination with high-intensity artificial sweeteners, in order to offset their low sweetness. Sugar alcohols are used widely in the food industry as thickeners and sweeteners. Since they contain multiple –OH groups, they are classified as polyols. They are white, water-soluble solids that can occur naturally or be produced industrially by hydrogenating sugars. Sugar alcohols (also called polyhydric alcohols, polyalcohols, alditols or glycitols) are organic compounds, typically derived from sugars, containing one hydroxyl group (–OH) attached to each carbon atom. It is 60–70% as sweet as sugar and almost noncaloric. The rest is one of two cyclic forms of glucose formed when the hydroxyl group on carbon 5 (C 5) bonds to the aldehyde carbon 1 (C 1), as shown below.Erythritol is a sugar alcohol. The linear form of glucose shown above makes up less than 3% of the glucose molecules in a water solution. The functional groups are the aldehyde and hydroxyl groups.īecause of these polar functional groups, glucose (and other monosaccharides) are highly soluble in water (1.5 g/mL at 25 ✬). Click on the mouse at left to clear the text and reset the image. One structure of glucose is shown below.Ĭlick on the step numbers below to see some important things about glucose's structure. Glucose is called a monosaccharideīecause it forms one simple building block of more complicated carbohydrates.
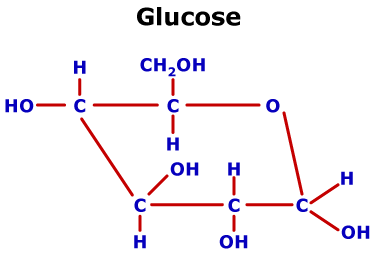
One of the most important carbohydrates in the body is glucose (C 6H 12O 6).


 0 kommentar(er)
0 kommentar(er)
